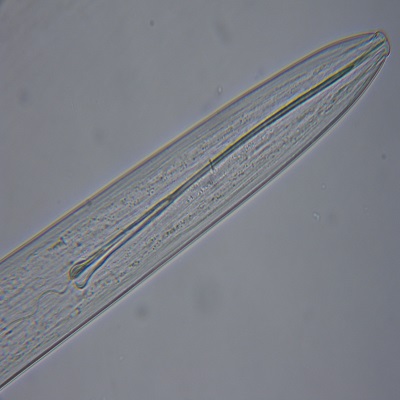
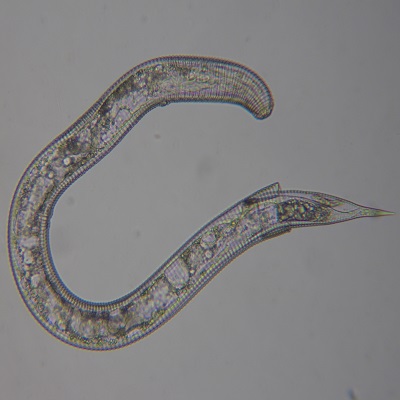


Extraction of the turfgrass rootzone to allow identification and population assessment of free-living turf parasitic nematodes. Root and other plant tissues will also be visually assessed for evidence of any endoparasitic nematode species. Results are generally provided in 2 to 3 days but when analysis takes longer, a preliminary result will be provided.
This analysis does not determine whether materials are ‘nematode free’. If a ‘nematode free’ analysis is required, please contact me.
Additional information
This analysis is ONLY for the assessment of free-living plant parasitic nematodes in amenity turfgrass areas or rootzones that have recently supported a turf sward. If you require other nematode analyses, please contact me. If you are able to email photographs of the developing symptoms, they will be very useful in determining the final diagnosis.
Free-living nematode populations are not evenly distributed across a turf area but tend to be patchy in their distribution (developing as ‘hot spots’ of high populations) and can also vary considerably with depth in the rootzone. If you would like to know whether plant parasitic nematodes are present in your managed turf area(s), a single sample composed of say 8 to 10, 15mm diameter cores, taken to just below the depth of the root development, will be suitable. Plant parasitic nematodes are obligate parasites and they need a living plant on which to feed. If the nematodes can’t obtain sufficient nutrition from a damaged plant, either, the nematode populations will decline, the nematodes will migrate deeper in to the rootzone or the nematodes will migrate away from the affected area to find more productive roots on which to feed. It is not uncommon to find high populations of certain nematode species beneath apparently healthy turf that surrounds symptomatic plants. However, endo-parasitic species can often be seen in heavily damaged turf.
This assessment physically extracts free living nematodes from the rootzone sample and separates them from any fine rootzone particles so that the nematodes can be observed by microscopy. The separation procedure relies on the nematodes actively moving through a physical barrier and takes 24h to complete. For this reason, any dead nematodes or eggs in the sample will not be separated from the fine rootzone particles and cannot be assessed.
Some plant parasitic nematode species, e.g. Stubby Root nematodes Trichodorus sp. and Paratrichodorus sp., are extremely sensitive to sudden impact which will kill the nematodes in the sample and therefore mean that they will not be detected in the assessment.
The root appearance can often indicate potential nematode activity. Galling of the roots, stunted root development or excessive root branching are common symptoms of nematode damage. If the assessed roots indicate a potential nematode-related problem but none of the expected nematode species are identified in the sample, a repeat sample may be requested to check the analysis.
If nematode populations in a specific area are to be assessed over time, it is important that the same area is sampled to the same depth in the rootzone, for every sample date.
If the received sample is not suitable for the requested analysis, you will be contacted so that the work can be discussed before any assessment is completed.
Please see additional information on sending samples for fungal disease analysis and current fees. If you are in any doubt about what to send, please contact me.
An obligate parasite is one that is dependent on a living host in order to obtain its nutrition. Plant parasitic nematodes are dependent on their host and do not feed on dead plants. They are also unlikely to feed on plants that have been weakened by fungal disease because the plant will not provide the required level of nutrition. However, nematode-stressed plants can be more susceptible to fungal disease than similar plants in nematode-free rootzones.